Which Statement Is Correct Concerning Animal Viruses?
Viral entry is the primeval stage of infection in the viral life cycle, as the virus comes into contact with the host cell and introduces viral textile into the cell. The major steps involved in viral entry are shown below.[1] Despite the variation among viruses, in that location are several shared generalities concerning viral entry.[2]
Reducing cellular proximity [edit]
How a virus enters a cell is dissimilar depending on the type of virus it is. A virus with a nonenveloped capsid enters the jail cell by attaching to the zipper factor located on a host cell. Information technology then enters the jail cell by endocytosis or by making a hole in the membrane of the host prison cell and inserting its viral genome.[two]


Cell entry by enveloped viruses is more complicated. Enveloped viruses enter the cell by attaching to an attachment cistron located on the surface of the host cell. They and so enter by endocytosis or a directly membrane fusion effect. The fusion effect is when the virus membrane and the host jail cell membrane fuse together allowing a virus to enter. It does this by attachment – or adsorption – onto a susceptible jail cell; a cell which holds a receptor that the virus tin demark to. The receptors on the viral envelope effectively go connected to complementary receptors on the cell membrane. This attachment causes the two membranes to remain in common proximity, favoring farther interactions betwixt surface proteins. This is also the first requisite that must be satisfied before a prison cell tin become infected. Satisfaction of this requisite makes the cell susceptible. Viruses that showroom this behavior include many enveloped viruses such as HIV and Canker simplex virus.[2]
These basic ideas extend to viruses that infect bacteria, known as bacteriophages (or but phages). Typical phages have long tails used to attach to receptors on the bacterial surface and inject their viral genome.
Overview [edit]
Prior to entry, a virus must adhere to a host cell. Attachment is achieved when specific proteins on the viral capsid or viral envelope bind to specific proteins chosen receptor proteins on the cell membrane of the target prison cell. A virus must now enter the jail cell, which is covered past a phospholipid bilayer, a jail cell's natural barrier to the outside earth. The process by which this barrier is breached depends upon the virus. Types of entry are:
- Membrane fusion or Hemifusion country : The prison cell membrane is punctured and made to farther connect with the unfolding viral envelope.
- Endocytosis : The host cell takes in the viral particle through the process of endocytosis, essentially engulfing the virus like information technology would a food particle.
- Viral penetration : The viral capsid or genome is injected into the host cell's cytoplasm.
Through the use of green fluorescent protein (GFP), virus entry and infection tin be visualized in existent-time. Once a virus enters a cell, replication is not immediate and indeed takes some time (seconds to hours).[3] [4]
Entry via membrane fusion [edit]
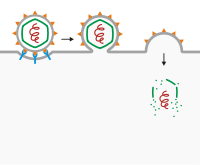
Viral entry via membrane fusion.
The most well-known example is through membrane fusion. In a number of viruses with a viral envelope, viral receptors adhere to the receptors on the surface of the cell and secondary receptors may be present to initiate the puncture of the membrane or fusion with the host cell. Post-obit attachment, the viral envelope fuses with the host cell membrane, causing the virus to enter. Viruses that enter a prison cell in this style included HIV, KSHV[five] [6] [7] [8] and herpes simplex virus.[9]
In SARS-CoV-two and like viruses, entry occurs through membrane fusion mediated past the spike protein, either at the cell surface or in vesicles. Inquiry efforts accept focused on the spike protein'southward interaction with its prison cell-surface receptor, angiotensin-converting enzyme 2 (ACE2). The evolved, high level of activity to mediate jail cell to prison cell fusion has resulted in an enhanced fusion capacity.[10] Inhibition of SARS-2 infection targets the spike proteins that harbor the capacity for membrane fusion.[11] Vaccinations are based on the inhibition of spike (S) glycoprotein mediating the fusion of the virus and its host cell membranes.[12] The fusion mechanism is likewise studied as a potential target for antiviral development.[13]
Entry via endocytosis [edit]

Viral entry via endocytosis.
Viruses with no viral envelope enter the cell by and large through endocytosis; they are ingested past the host cell through the cell membrane. Cells tin take in resources from the surroundings outside of the jail cell, and these mechanisms may be used by viruses to enter a prison cell in the same manner as ordinary resource. Once within the prison cell, the virus leaves the vesicle by which information technology was taken up in lodge to gain access to the cytoplasm. Examples include the poliovirus, Hepatitis C virus,[fourteen] and Foot-and-rima oris illness virus.[15]
Many enveloped viruses, such equally SARS-CoV-2, besides enter the cell through endocytosis. Entry via the endosome guarantees low pH and exposure to proteases which are needed to open the viral capsid and release the genetic textile inside. Further, endosomes send the virus through the cell and ensure that no trace of the virus is left on the surface, which could exist a substrate for allowed recognition.[16]
Entry via genetic injection [edit]
A third and more than specific case, is by simply attaching to the surface of the jail cell via receptors on the cell, and injecting only its genome into the prison cell, leaving the remainder of the virus on the surface. This is restricted to viruses in which only the genome is required for infection of a cell (for case positive-strand RNA viruses considering they tin be immediately translated) and farther restricted to viruses that actually exhibit this behavior. The best studied example includes the bacteriophages; for example, when the tail fibers of the T2 phage land on a jail cell, its central sheath pierces the prison cell membrane and the phage injects Deoxyribonucleic acid from the head capsid directly into the cell.[17]
Aftermath [edit]
In one case a virus is in a cell, information technology volition activate formation of proteins (either by itself or using the host) to gain total control of the host cell, if information technology is able to. Control mechanisms include the suppression of intrinsic jail cell defenses, suppression of cell signaling and suppression of host cellular transcription and translation. Often, it is these cytotoxic effects that lead to the decease and decline of a jail cell infected by a virus.
A jail cell is classified equally susceptible to a virus if the virus is able to enter the cell. Afterward the introduction of the viral particle, unpacking of the contents (viral proteins in the tegument and the viral genome via some class of nucleic acid) occurs as preparation of the next stage of viral infection: viral replication.
References [edit]
- ^ Subramanian RP, Geraghty RJ (20 February 2007). "Herpes simplex virus blazon 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B". Proceedings of the National Academy of Sciences, USA. 104 (viii): 2903–08. Bibcode:2007PNAS..104.2903S. doi:10.1073/pnas.0608374104. PMC1815279. PMID 17299053.
- ^ a b c "Virus entry into host jail cell ~ ViralZone page". viralzone.expasy.org . Retrieved 2021-02-05 .
- ^ Lakadamyali, Melike; Michael J. Rust; Hazen P. Babcock; Xiaowei Zhuang (2003). "Visualizing infection of individual influenza viruses". Proceedings of the National Academy of Sciences of the Us. 100 (16): 9280–85. Bibcode:2003PNAS..100.9280L. doi:10.1073/pnas.0832269100. PMC170909. PMID 12883000.
- ^ Joo, 1000-I; P Wang (2008-05-fifteen). "Visualization of Targeted Transduction by Engineered Lentiviral Vectors". Factor Ther. 15 (xx): 1384–96. doi:10.1038/gt.2008.87. ISSN 0969-7128. PMC2575058. PMID 18480844.
- ^ Kumar, Binod; Chandran, Bala (November 14, 2016). "KSHV Entry and Trafficking in Target Cells-Hijacking of Cell Point Pathways, Actin and Membrane Dynamics". Viruses. viii (eleven): 305. doi:10.3390/v8110305. ISSN 1999-4915. PMC5127019. PMID 27854239.
- ^ Kumar, Binod; Dutta, Dipanjan; Iqbal, Jawed; Ansari, Mairaj Ahmed; Roy, Arunava; Chikoti, Leela; Pisano, Gina; Veettil, Mohanan Valiya; Chandran, Bala (October 2016). "ESCRT-I Protein Tsg101 Plays a Role in the Post-macropinocytic Trafficking and Infection of Endothelial Cells by Kaposi'due south Sarcoma-Associated Herpesvirus". PLOS Pathogens. 12 (10): e1005960. doi:10.1371/journal.ppat.1005960. ISSN 1553-7374. PMC5072609. PMID 27764233.
- ^ Veettil, Mohanan Valiya; Kumar, Binod; Ansari, Mairaj Ahmed; Dutta, Dipanjan; Iqbal, Jawed; Gjyshi, Olsi; Bottero, Virginie; Chandran, Bala (Apr 2016). "ESCRT-0 Component Hrs Promotes Macropinocytosis of Kaposi'southward Sarcoma-Associated Herpesvirus in Homo Dermal Microvascular Endothelial Cells". Journal of Virology. 90 (8): 3860–72. doi:10.1128/JVI.02704-xv. ISSN 1098-5514. PMC4810545. PMID 26819309.
- ^ Khanna, Madhu; Sharma, Sachin; Kumar, Binod; Rajput, Roopali (2014). "Protective Immunity Based on the Conserved Hemagglutinin Stem Domain and Its Prospects for Universal Influenza Vaccine Evolution". BioMed Enquiry International. 2014: 546274. doi:10.1155/2014/546274. ISSN 2314-6133. PMC4055638. PMID 24982895.
- ^ Campadelli-Fiume, Gabriella; Amasio, Michele; Avitabile, Elisa; Cerretani, Arianna; Forghieri, Cristina; Gianni, Tatiana; Menotti, Laura (2007). "The multipartite organisation that mediates entry of herpes simplex virus into the cell". Reviews in Medical Virology. 17 (5): 313–326. doi:10.1002/rmv.546. ISSN 1052-9276. PMID 17573668. S2CID 30771615.
- ^ Zhu, Yuanmei; Yu, Danwei; Yan, Hongxia; Chong, Huihui; He, Yuxian (2020). "Blueprint of Stiff Membrane Fusion Inhibitors against SARS-CoV-2, an Emerging Coronavirus with High Fusogenic Activity". Periodical of Virology. 94 (14). doi:10.1128/JVI.00635-20. PMC7343218. PMID 32376627.
- ^ Xia, Shuai; Liu, Meiqin; Wang, Chao; Xu, Wei; Lan, Qiaoshuai; Feng, Siliang; Qi, Feifei; Bao, Linlin; Du, Lanying; Liu, Shuwen; Qin, Chuan (2020-03-30). "Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a loftier capacity to mediate membrane fusion". Cell Inquiry. thirty (4): 343–355. doi:10.1038/s41422-020-0305-ten. ISSN 1001-0602. PMC7104723. PMID 32231345.
- ^ Outlaw, Victor K.; Bovier, Francesca T.; Mears, Megan C.; Cajimat, Maria North.; Zhu, Yun; Lin, Michelle J.; Addetia, Amin; Lieberman, Nicole A. P.; Peddu, Vikas; Xie, Xuping; Shi, Pei-Yong (2020-x-xx). "Inhibition of Coronavirus Entry In Vitro and Ex Vivo by a Lipid-Conjugated Peptide Derived from the SARS-CoV-2 Spike Glycoprotein HRC Domain". mBio. 11 (v). doi:ten.1128/mBio.01935-xx. ISSN 2150-7511. PMC7587434. PMID 33082259.
- ^ Tang, Tiffany; Bidon, Miya; Jaimes, Javier A.; Whittaker, Gary R.; Daniel, Susan (June 2020). "Coronavirus membrane fusion mechanism offers a potential target for antiviral development". Antiviral Research. 178: 104792. doi:x.1016/j.antiviral.2020.104792. ISSN 0166-3542. PMC7194977. PMID 32272173.
- ^ Helle F, Dubuisson J. "Hepatitis C virus entry into host cells." Prison cell Mol Life Sci. 2007 Oct iv
- ^ N.J. Dimmock et al. Introduction to Mod Virology, 6th edition." Blackwell Publishing, 2007.[ ISBN missing ] [ page needed ]
- ^ Howley, Peter M; Knipe, David G Fields Virology [ page needed ] Lippincott Williams & Williams 2013[ ISBN missing ]
- ^ Sebestyén, Magdolna K.; Budker, Vladimir G.; Budker, Tatiana; Subbotin, Vladimir Thousand.; Zhang, Guofeng; Monahan, Sean D.; Lewis, David 50.; Wong, Then C.; Hagstrom, James Due east.; Wolff, Jon A. (July 2006). "Machinery of plasmid delivery past hydrodynamic tail vein injection. I. Hepatocyte uptake of various molecules". The Periodical of Factor Medicine. 8 (seven): 852–873. doi:x.1002/jgm.921. ISSN 1099-498X. PMID 16724360. S2CID 564796.
Source: https://en.wikipedia.org/wiki/Viral_entry
Posted by: levittaphism.blogspot.com

0 Response to "Which Statement Is Correct Concerning Animal Viruses?"
Post a Comment